Lewis Dot Diagram For Ar
Representing Valence Electrons in Lewis Symbols
Lewis symbols use dots to visually represent the valence electrons of an atom.
Learning Objectives
Retrieve the Lewis structure formalism for representing valance electrons
Primal Takeaways
Key Points
- Electrons be outside of an atom 's nucleus and are plant in principal energy levels that contain only upward to a specific number of electrons.
- The outermost main free energy level that contains electrons is called the valence level and contains valence electrons.
- Lewis symbols are diagrams that evidence the number of valence electrons of a particular chemical element with dots that represent lone pairs.
- Lewis symbols practise non visualize the electrons in the inner principal energy levels.
Primal Terms
- main energy levels: The different levels where electrons can be constitute and that occur at specific distances from the cantlet'southward nucleus. Each level is associated with a particular energy value that electrons within it accept.
- valence level: The outermost principal energy level, which is the level furthest away from the nucleus that still contains electrons.
- valence electrons: The electrons of atoms that participate in the germination of chemic bonds.
- Lewis symbols: Symbols of the elements with their number of valence electrons represented as dots
Lewis symbols (as well known equally Lewis dot diagrams or electron dot diagrams) are diagrams that represent the valence electrons of an atom. Lewis structures (also known as Lewis dot structures or electron dot structures) are diagrams that stand for the valence electrons of atoms inside a molecule. These Lewis symbols and Lewis structures help visualize the valence electrons of atoms and molecules, whether they exist equally alone pairs or inside bonds.
Principal Energy Levels
An atom consists of a positively charged nucleus and negatively charged electrons. The electrostatic allure between them keeps electrons 'spring' to the nucleus so they stay within a certain distance of it. Careful investigations have shown that not all electrons within an atom accept the same average position or energy. We say the electrons 'reside' in different primary energy levels, and these levels be at different radii from the nucleus and take rules regarding how many electrons they can arrange.
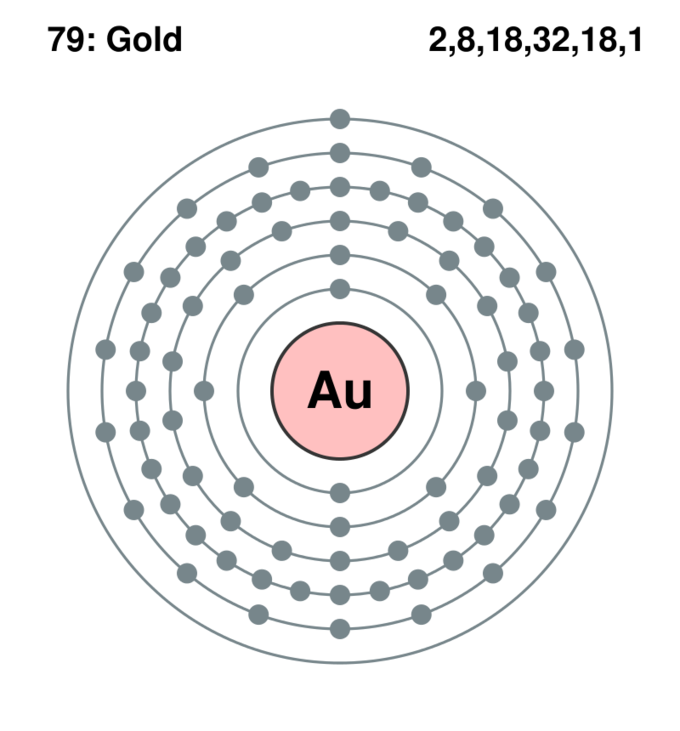
Principal energy levels of aureate (Au): The figure shows the organization of the electrons around the nucleus of a golden (Au) atom. Notice that the showtime energy level (closest to the nucleus) can have just 2 electrons, while more electrons tin can 'fit' within a given level further out. The number of electrons in each level is listed on the upper right corner of the figure. Notice that the outermost level has only ane electron.
Every bit an example, a neutral cantlet of aureate (Au) contains 79 protons in its nucleus and 79 electrons. The commencement principal energy level, which is the ane closest to the nucleus, can concur a maximum of ii electrons. The 2nd principal energy level can have 8, the third tin can have 18, and and so on, until all 79 electrons have been distributed.
The outermost master free energy level is of great interest in chemistry because the electrons it holds are the furthest away from the nucleus, and therefore are the ones about loosely held by its attractive forcefulness; the larger the distance betwixt ii charged objects, the smaller the strength they exert on each other. Chemical reactivity of all of the different elements in the periodic tabular array depends on the number of electrons in that last, outermost level, called the valence level or valence vanquish. In the case of gold, there is just one valence electron in its valence level.
Octet of Valence Electrons
Atoms proceeds, lose, or share electrons in their valence level in order to achieve greater stability, or a lower free energy state. From this perspective, bonds betwixt atoms form and then that the bonded atoms are in a lower energy state compared to when they were past themselves. Atoms tin reach this more stable state by having a valence level which contains equally many electrons as it tin can hold. For the start principal energy level, having two electrons in it is the almost stable arrangement, while for all other levels exterior of the showtime, eight electrons are necessary to achieve the most stable state.
Lewis Symbols
In the Lewis symbol for an cantlet, the chemic symbol of the chemical element (as found on the periodic table) is written, and the valence electrons are represented equally dots surrounding it. Only the electrons in the valence level are shown using this notation. For example, the Lewis symbol of carbon depicts a "C' surrounded by iv valence electrons because carbon has an electron configuration of 1sii2s22p2.

The Lewis symbol for carbon: Each of the 4 valence electrons is represented equally a dot.
Electrons that are non in the valence level are not shown in the Lewis symbol. The reason for this is that the chemic reactivity of an atom of the element is solely determined by the number of its valence electrons, and not its inner electrons. Lewis symbols for atoms are combined to write Lewis structures for compounds or molecules with bonds between atoms.
Writing Lewis Symbols for Atoms
The Lewis symbol for an atom depicts its valence electrons every bit dots around the symbol for the chemical element.
Learning Objectives
Write Lewis symbols for atoms
Cardinal Takeaways
Key Points
- The columns, or groups, in the periodic table are used to determine the number of valence electrons for each element.
- The noble/ inert gases are chemically stable and take a total valence level of electrons.
- Other elements react in order to achieve the same stability as the noble gases.
- Lewis symbols represent the valence electrons as dots surrounding the elemental symbol for the atom.
Key Terms
- group: A column in the periodic table that consists of elements with similar chemical reactivity, because they have the same number of valence electrons.
- Noble Gases: Inert, or unreactive, elements in the last group in the periodic table which are typically constitute in the gaseous form.
- Lewis symbol: Formalism in which the valence electrons of an atom are represented as dots.
Determining the Number of Valence Electrons
In order to write the Lewis symbol for an atom, you must first determine the number of valence electrons for that element. The organisation of the periodic tabular array can assist y'all figure out this information. Since we have established that the number of valence electrons determines the chemic reactivity of an element, the table orders the elements by number of valence electrons.
Each column (or group) of the periodic table contains elements that have the same number of valence electrons. Furthermore, the number of columns (or groups) from the left edge of the tabular array tells us the exact number of valence electrons for that element. Call back that any valence level can accept upward to eight electrons, except for the first master energy level, which can simply take two.

Periodic table of the elements: Group numbers shown by Roman numerals (in a higher place the tabular array) tell usa how many valence electrons there are for each chemical element.
Some periodic tables list the group numbers in Arabic numbers instead of Roman numerals. In that instance, the transition metal groups are included in the counting and the groups indicated at the tiptop of the periodic table have numbers 1, 2, 13, 14, 15, 16, 17, 18. The corresponding roman numerals used are I, Ii, III, IV, 5, VI, Vii, 8.
Survey of the Groups in the Periodic Table
Take the first column or group of the periodic tabular array (labeled 'I'): hydrogen (H), lithium (Li), sodium (Na), potassium (Thousand), etc. Each of these elements has one valence electron. The 2nd column or grouping (labeled 'Two') means that beryllium (Exist), magnesium (Mg), calcium (Ca), etc., all have two valence electrons.
The middle part of the periodic table that contains the transition metals is skipped in this process for reasons having to exercise with the electronic configuration of these elements.
Proceeding to the cavalcade labeled 'III', we observe that those elements (B, Al, Ga, In,...) have three valence electrons in their outermost or valence level.
Nosotros can continue this inspection of the groups until nosotros reach the eighth and last column, in which the near stable elements are listed. These are all gaseous under normal conditions of temperature and pressure, and are called 'noble gases.' Neon (Ne), argon (Ar), krypton (Kr), etc., each incorporate eight electrons in their valence level. Therefore, these elements accept a full valence level that has the maximum number of electrons possible. Helium (He), at the very top of this column is an exception considering it has two valence electrons; its valence level is the outset chief free energy level which can only accept two electrons, and then it has the maximum number of electrons in its valence level every bit well.
The Lewis symbol for helium: Helium is ane of the noble gases and contains a full valence beat. Unlike the other noble gases in Group eight, Helium just contains two valence electrons. In the Lewis symbol, the electrons are depicted as two lone pair dots.
The noble gases represent elements of such stability that they are not chemically reactive, so they can be called inert. In other words, they don't need to bond with any other elements in order to attain a lower free energy configuration. We explicate this phenomenon by attributing their stability to having a 'full' valence level.
The significance in understanding the nature of the stability of noble gases is that it guides us in predicting how other elements will react in social club to accomplish the same electronic configuration as the noble gases by having a total valence level.
Writing Lewis Symbols for Atoms
Lewis symbols for the elements describe the number of valence electrons equally dots. In accord with what we discussed above, here are the Lewis symbols for the outset twenty elements in the periodic table. The heavier elements will follow the same trends depending on their group.
Once you lot can draw a Lewis symbol for an cantlet, you can use the knowledge of Lewis symbols to create Lewis structures for molecules.
Introduction to Lewis Structures for Covalent Molecules
In covalent molecules, atoms share pairs of electrons in social club to achieve a full valence level.
Learning Objectives
Predict and draw the Lewis structure of elementary covalent molecules and compounds
Key Takeaways
Key Points
- The octet rule says that the noble gas electronic configuration is a particularly favorable one that can be accomplished through formation of electron pair bonds between atoms.
- In many atoms, non all of the electron pairs comprising the octet are shared betwixt atoms. These unshared, non-bonding electrons are chosen ' lone pairs ' of electrons.
- Although lone pairs are not directly involved in bond formation, they should always be shown in Lewis structures.
- There is a logical procedure that can be followed to draw the Lewis structure of a molecule or chemical compound.
Key Terms
- octet rule: Atoms endeavour to achieve the electronic configuration of the noble gas nearest to them in the periodic tabular array by achieving a full valence level with eight electrons.
- exceptions to the octet dominion: Hydrogen (H) and helium (He) only need two electrons to have a full valence level.
- covalent bail: 2 atoms share valence electrons in order to achieve a noble gas electronic configuration.
- Lewis structure: Formalism used to show the structure of a molecule or chemical compound, in which shared electrons pairs between atoms are indicated by dashes. Non-bonding, lone pairs of electrons must also be shown.
The Octet Dominion
Noble gases like He, Ne, Ar, Kr, etc., are stable considering their valence level is filled with as many electrons as possible. Eight electrons fill up the valence level for all noble gases, except helium, which has two electrons in its total valence level. Other elements in the periodic table react to grade bonds in which valence electrons are exchanged or shared in social club to attain a valence level which is filled, just like in the noble gases. We refer to this chemical trend of atoms as 'the octet rule,' and information technology guides u.s. in predicting how atoms combine to form molecules and compounds.
Covalent Bonds and Lewis Diagrams of Simple Molecules
The simplest case to consider is hydrogen (H), which is the smallest element in the periodic table with one proton and i electron. Hydrogen can become stable if it achieves a total valence level similar the noble gas that is closest to it in the periodic table, helium (He). These are exceptions to the octet rule because they only crave two electrons to have a total valence level.
2 H atoms tin can come together and share each of their electrons to create a ' covalent bond.' The shared pair of electrons can be thought of equally belonging to either atom, and thus each atom now has 2 electrons in its valence level, similar He. The molecule that results is H2, and it is the almost arable molecule in the universe.
Lewis structure of diatomic hydrogen: This is the process through which the H2 molecule is formed. Two H atoms, each contributing an electron, share a pair of electrons. This is known as a 'single covalent bond.' Observe how the ii electrons tin can exist found in a region of space between the two diminutive nuclei.
The Lewis formalism used for the H2 molecule is H:H or H—H. The old, known as a 'Lewis dot diagram,' indicates a pair of shared electrons betwixt the diminutive symbols, while the latter, known as a 'Lewis structure,' uses a dash to signal the pair of shared electrons that class a covalent bail. More complicated molecules are depicted this fashion as well.
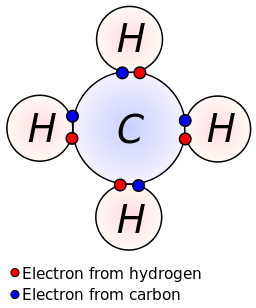
Lewis dot dragram for methane: Methyl hydride, with molecular formula CH4, is shown. The electrons are color-coded to indicate which atoms they belonged to earlier the covalent bonds formed, with blood-red representing hydrogen and bluish representing carbon. Iv covalent bonds are formed so that C has an octet of valence electrons, and each H has two valence electrons—i from the carbon cantlet and one from one of the hydrogen atoms.
At present consider the example of fluorine (F), which is found in group 7 (or 17) of the periodic table. It therefore has seven valence electrons and only needs 1 more in lodge to accept an octet. One mode that this can happen is if two F atoms make a bond, in which each atom provides ane electron that can exist shared betwixt the two atoms. The resulting molecule that is formed is Ftwo, and its Lewis construction is F—F.
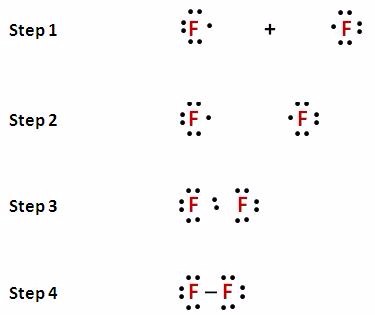
Achieving an octet of valence electrons: 2 fluorine atoms are able to share an electron pair, which becomes a covalent bail. Notice that just the outer (valence level) electrons are involved, and that in each F atom, 6 valence electrons exercise non participate in bonding. These are 'lone pairs' of electrons.
Later on a bond has formed, each F atom has 6 electrons in its valence level which are not used to form a bail. These non-bonding valence electrons are called 'lone pairs' of electrons and should always be indicated in Lewis diagrams.
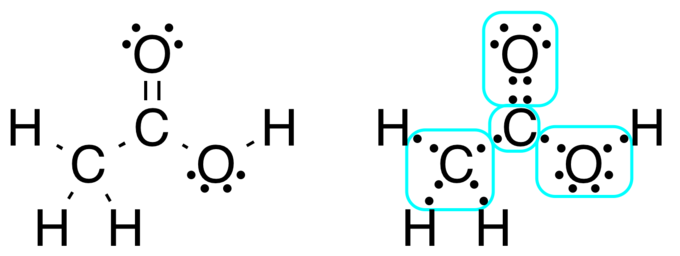
Lewis structure of acerb acrid: Acetic acid, CH3COOH, tin can be written out with dots indicating the shared electrons, or, preferably, with dashes representing covalent bonds. Notice the lone pairs of electrons on the oxygen atoms are still shown. The methyl group carbon cantlet has 6 valence electrons from its bonds to the hydrogen atoms considering carbon is more electronegative than hydrogen. Besides, one electron is gained from its bond with the other carbon atom because the electron pair in the C−C bond is split equally.
Procedure for Drawing Simple Lewis Structures
We have looked at how to determine Lewis structures for unproblematic molecules. The procedure is as follows:
- Write a structural diagram of the molecule to clearly show which atom is continued to which (although many possibilities exist, nosotros usually pick the element with the well-nigh number of possible bonds to be the central cantlet).
- Draw Lewis symbols of the individual atoms in the molecule.
- Bring the atoms together in a way that places viii electrons around each cantlet (or 2 electrons for H, hydrogen) wherever possible.
- Each pair of shared electrons is a covalent bail which tin be represented by a dash.

Alternate view of lewis dot construction of h2o: This arrangement of shared electrons between O and H results in the oxygen atom having an octet of electrons, and each H atom having two valence electrons.
Multiple bonds tin as well form betwixt elements when two or three pairs of electrons are shared to produce double or triple bonds, respectively. The Lewis structure for carbon dioxide, COii, is a good case of this.

Lewis construction of carbon dioxide: This effigy explains the bonding in a CO2 molecule. Each O atom starts out with six (ruddy) electrons and C with 4 (black) electrons, and each bond behind an O atom and the C atom consists of two electrons from the O and two of the four electrons from the C.
In guild to achieve an octet for all 3 atoms in CO2, two pairs of electrons must be shared between the carbon and each oxygen. Since 4 electrons are involved in each bond, a double covalent bond is formed. You lot can see that this is how the octet dominion is satisfied for all atoms in this case. When a double bail is formed, you still need to evidence all electrons, so double dashes between the atoms evidence that four electrons are shared.
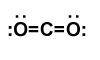
Final Lewis structure for carbon dioxide: Covalent bonds are indicated as dashes and lone pairs of electrons are shown as pairs of dots. in carbon dioxide, each oxygen atom has two lone pairs of electrons remaining; the covalent bonds between the oxygen and carbon atoms each utilize two electrons from the oxygen atom and two from the carbon.
Lewis Structures for Polyatomic Ions
The Lewis construction of an ion is placed in brackets and its charge is written as a superscript outside of the brackets, on the upper right.
Learning Objectives
Apply the rules for drawing Lewis structures to polyatomic ions
Key Takeaways
Key Points
- Ions are treated well-nigh the same mode equally a molecule with no charge. All the same, the number of electrons must be adjusted to account for the net electrical charge of the ion.
- When counting electrons, negative ions should have extra electrons placed in their Lewis structures, while positive ions should have fewer electrons than an uncharged molecule.
Fundamental Terms
- polyatomic ion: A charged species composed of 2 or more than atoms covalently bonded, or of a metal complex that acts as a single unit in acid-base chemistry or in the formation of salts. Likewise known as a molecular ion.
The total number of electrons represented in a Lewis structure is equal to the sum of the numbers of valence electrons in each individual atom. Non-valence electrons are not represented in Lewis structures. After the total number of available electrons has been determined, electrons must exist placed into the construction.
Lewis structures for polyatomic ions are drawn by the same methods that we accept already learned. When counting electrons, negative ions should take extra electrons placed in their Lewis structures; positive ions should accept fewer electrons than an uncharged molecule. When the Lewis construction of an ion is written, the entire structure is placed in brackets, and the charge is written as a superscript on the upper right, outside of the brackets. For instance, consider the ammonium ion, NH4 +, which contains 9 (v from N and 1 from each of the 4 H atoms) –1 = 8 electrons. 1 electron is subtracted considering the unabridged molecule has a +1 charge.
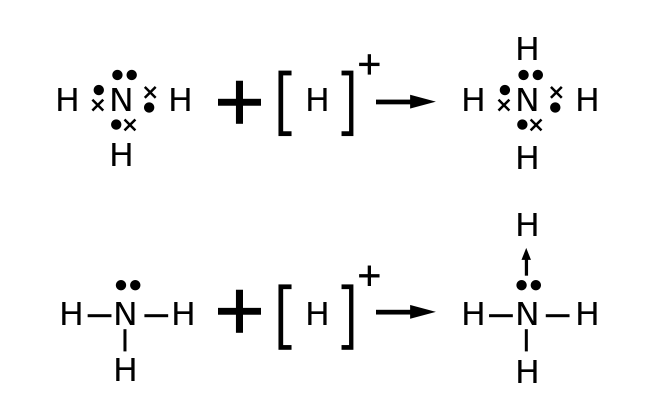
Coordinate covalent bonding: The ammonium ion, NH4+, contains 9–1 = eight electrons.
Negative ions follow the aforementioned procedure. The chlorite ion, ClOii –, contains 19 (7 from the Cl and 6 from each of the two O atoms) +1 = twenty electrons. Ane electron is added because the entire molecule has a -1 charge.

Hypochlorite ion Lewis structure: The hypochlorite ion, ClO−, contains 13 + i = 14 electrons.
Licenses and Attributions
Lewis Dot Diagram For Ar,
Source: https://www.coursehero.com/study-guides/trident-boundless-chemistry/lewis-dot-symbols-and-lewis-structures/
Posted by: hagertywiced1936.blogspot.com


0 Response to "Lewis Dot Diagram For Ar"
Post a Comment